According to Kremlin propagandists, any signs of clinical trials being conducted on the territory of Ukraine are evidence of “secret and dangerous experiments for patients.” However, clinical trials are not conducted secretly; on the contrary, they are conducted openly and with the patient’s consent.
With the support of the USAID Health Reform Support project, VoxCheck analyzes and refutes public health narratives spread in the information space of Ukraine, Belarus, and russia on a weekly basis.
Russian propagandists once again claim that secret drug trials were conducted on the territory of Ukraine, including at Psychiatric Hospital No. 7 in Mariupol. Moreover, these drugs allegedly could cause various forms of cancer. The focus is on the testing of the experimental drug SB4 for the treatment of rheumatoid arthritis. As evidence, they provide documents purportedly obtained by the Russian state media “RIA Novosti.”
Screenshot of the post
What’s the reality?
Earlier, VoxCheck had already debunked disinformation about Ukrainian doctors illegally testing the drug SB4-G31-RA on residents of Mariupol. The research was indeed conducted from May 2013 to November 2014, but it was not secret; it was open. During the study, experts assessed the effectiveness and safety of the drug for patients with moderate to severe rheumatoid arthritis compared to another drug, Enbrel.
Currently, SB4 is approved by the U.S. Food and Drug Administration (FDA) and is available on the American market under the name Eticovo. The drug is also approved on many other markets under the names Benepali and Brenzys. SB4 received its first approval in South Korea in 2015, and in 2016, it was approved in the European Union, Canada, and Australia.
In addition to Ukraine, clinical trials of the drug were also conducted in Bulgaria, Colombia, Czechia, Hungary, India, South Korea, Lithuania, Mexico, Poland, and the United Kingdom. All clinical trials, including this one, have strict regulations. In particular, the European Union registry website stated that Independent Ethics Committee (IEC) and Institutional Review Boards (IRB) reviewed and approved the SB4-G31-RA study protocols at each research center. As indicated, the studies were conducted in accordance with the ethical principles of the Helsinki Declaration (2008), as well as in accordance with the national legislation of the participating countries. Each patient signed informed consent and received a verbal explanation of the nature and purpose of the study. The IEC and IRB reviewed and approved the consent documents signed by the study participants.
Details about the research conducted in Ukraine can be found on the website of the State Expert Center of the Ministry of Health of Ukraine. For example, in the case of SB4, it involves Phase III clinical trials, where a large group of patients (hundreds or even more) is involved to determine the effectiveness of treatment and identify side effects. It is after this stage that a decision is made regarding the registration of the drug.
Clinical trials were not conducted on the premises of Psychiatric Hospital No. 7 in Mariupol. Instead, individual medical institutions in Kharkiv, Donetsk, Vinnytsia, Kyiv, Zaporizhzhia, Poltava, Ivano-Frankivsk, and Simferopol participated in the trials. None of them are psychiatric hospitals. According to the Law “On Medicines,” only capable individuals can participate in clinical trials. People whose legal capacity is limited due to mental illness can only participate in studies of drugs intended to treat corresponding conditions.
Regarding whether the drugs in question cause cancer, isolated cases of cancer were indeed recorded among patients. Out of 299 individuals who tested the SB4 drug, 18 people (6%) experienced serious side effects. In the group receiving Enbrel, comprising 297 individuals, 15 people (5%) experienced serious adverse reactions. In total, two cases of breast cancer development were recorded, causally related to treatment, in one case with SB4 and in the other with Enbrel.
The results are insufficient to claim that SB4 or Enbrel causes cancer. The most common side effects (over 20%) during the trials were injection site reactions (bleeding, redness, itching, pain, and swelling) and infections (cold, lung infections, bladder and skin infections).
The U.S. Food and Drug Administration (FDA) adds a warning that over the years, some patients taking these drugs have developed lymphoma. Typically, these were individuals with severe rheumatoid arthritis.
It’s important to understand that these medications are prescribed to patients with moderate to severe rheumatoid arthritis to alleviate symptoms, often when other treatments have not been effective. The risks associated with using the drug are assessed individually by the doctor for each patient. Additionally, patients prescribed SB4 or Enbrel receive a special card with detailed information about their safety. This card helps patients recognize any serious side effects and understand when they need to seek immediate medical attention.
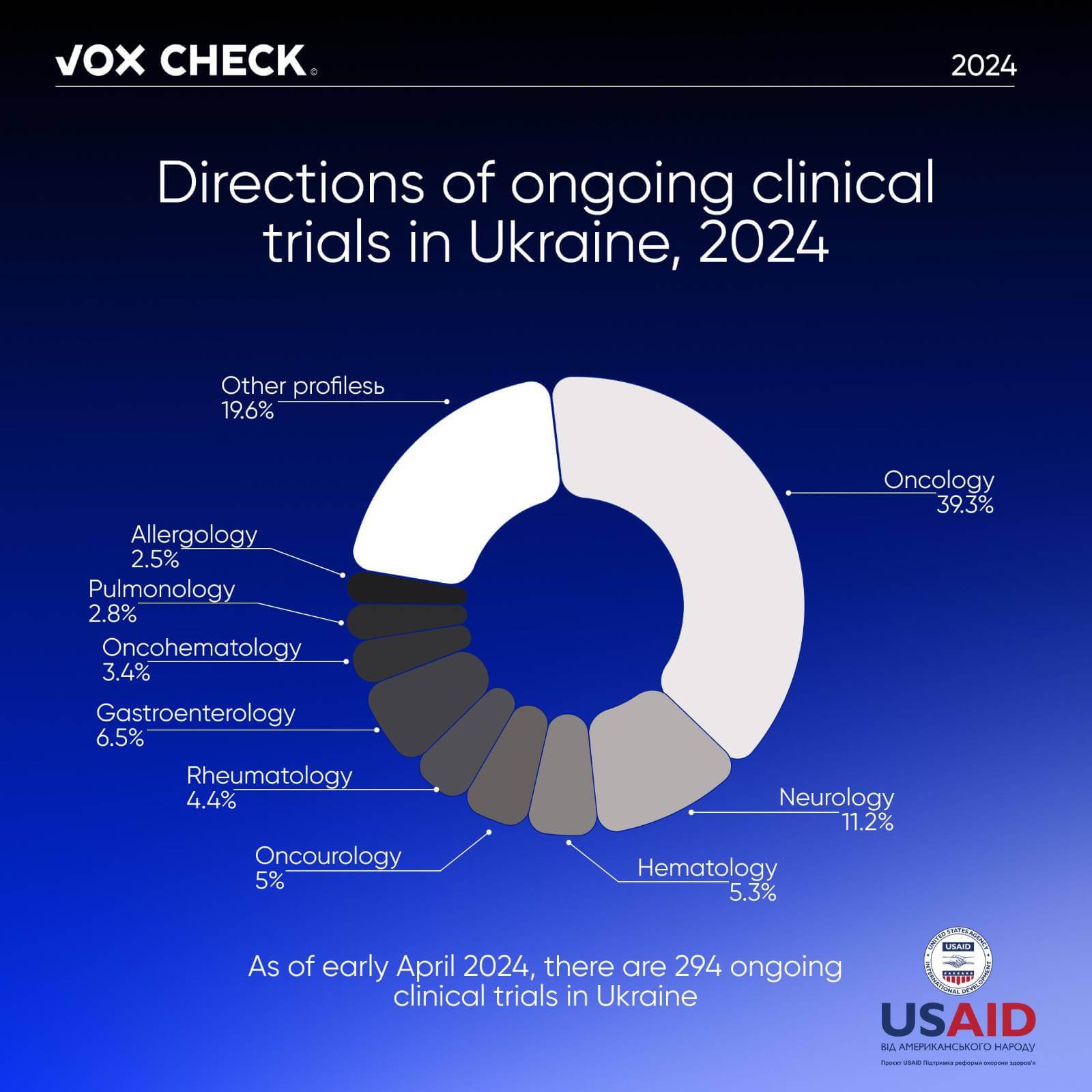
As of early April 2024, there are 294 ongoing clinical trials in Ukraine. According to the Ministry of Health of Ukraine, participation in a clinical trial provides the following benefits to patients:
- Free treatment: Patients are provided not only with investigational drugs but also with medications for supportive and basic therapy.
- Regular examinations and monitoring.
- Access to innovative drugs: Such drugs are often the only hope for survival, for example, for oncology patients at stages when no other treatment method is effective.
- High level of protection: Participation in the study is voluntary. After signing an informed consent form, the patient receives insurance for the duration of the study and has the right to withdraw from it at any time.
This information piece was produced with the assistance of the United States Agency for International Development (USAID), provided on behalf of the people of the United States of America. This article’s content, which does not necessarily reflect the views of USAID, the United States Government, is the sole responsibility of Deloitte Consulting under contract #72012118C00001.
Attention
The author doesn`t work for, consult to, own shares in or receive funding from any company or organization that would benefit from this article, and have no relevant affiliations