The 258th round of the Reform Index includes four reforms, three of which focus on the pharmaceutical market.
As part of the presidential initiative to lower drug prices, the government introduced new restrictions for pharmaceutical manufacturers and pharmacies. Our experts assessed this move negatively, making the resolution the first reform setback of 2025. In addition, the government authorized the use of patented medicines without the consent of patent holders in cases of shortage.
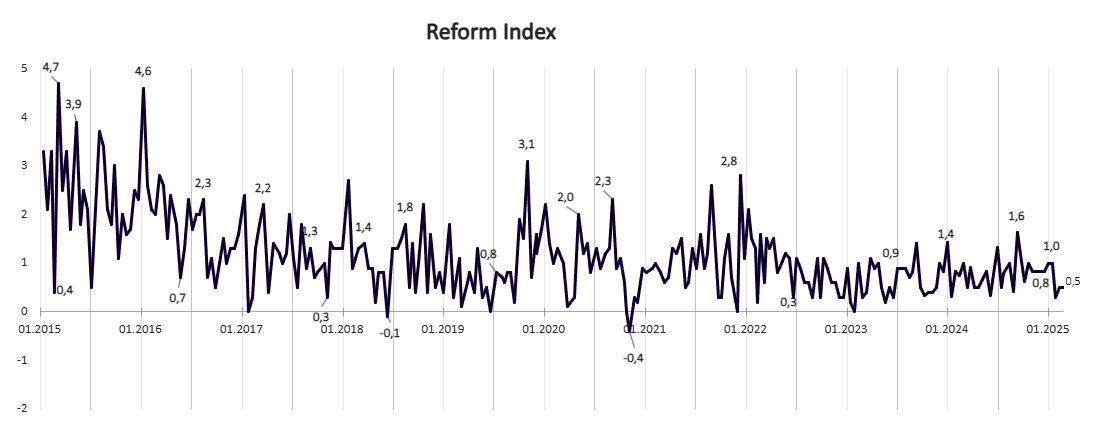
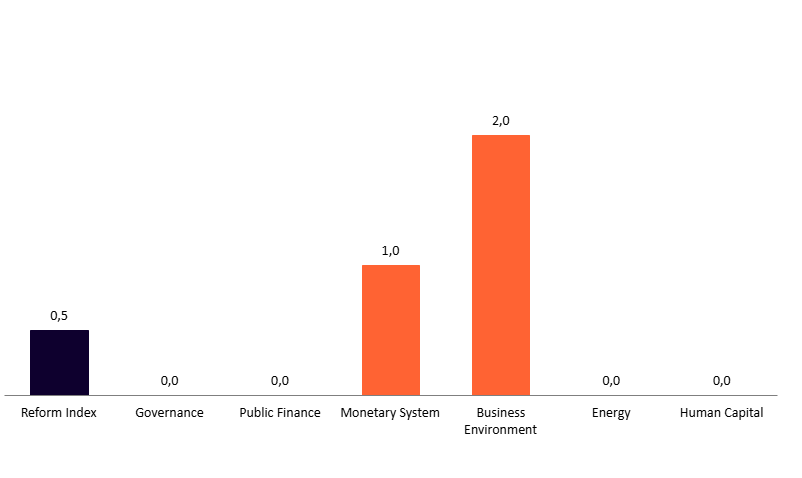
The 258th round covers reforms adopted between February 10 and February 23. The overall Reform Index score for this period is 0.5 points — the same as in the previous issue.
Graph 1. Dynamics of the Reform Index
Graph 2. Values of the Reform Index and its Components in the Current Assessment Round
Maximum retail markup on medicines set at 35%, –1 point
Starting March 1, 2025, the government began regulating markups on all medicines without exception. Previously, state price controls applied only to drugs on the National List of Essential Medicines and those purchased with public funds.
Under the new rules, the maximum allowable markup for suppliers is set at 8%. Pharmacies can apply a markup of up to 35% for over-the-counter drugs and between 10% and 25% for prescription medications and drugs from the National List. To enforce the new rules, the State Service for Food Safety and Consumer Protection will resume both scheduled and unscheduled inspections.
The resolution also bans pharmacies from entering into marketing agreements — arrangements under which manufacturers offer additional discounts on medicines. Previously, these agreements allowed pharmacies to provide discounts on popular drugs and maintain a broader range of medications, including those that are rarely purchased.
Information about the Reforms Index project, the list of Index experts and the database of the regulations assessed are available here.
Expert commentary
Oksana Kuziakiv, Executive Director at the Institute for Economic Research
“Cabinet Resolution No. 168, which came into force on March 1, 2025, introduced new markup limits on pharmaceuticals. While the measure aims to improve access to medicines for the population, it has raised concerns within the pharmaceutical industry due to its potential impact on the market and commercial dynamics.
The resolution establishes maximum markups — up to 35% above the purchase price for over-the-counter drugs, and a separate regressive scale for prescription medicines. Distributor markups must not exceed 8% of the wholesale price. The resolution also prohibits marketing and promotional services until the Cabinet clearly defines the rules for pharmaceutical marketing. A key provision is the resumption of both scheduled and unscheduled inspections by the State Service of Ukraine on Food Safety and Consumer Protection (SSUFSCP) to enforce pricing compliance.
These government measures have sparked concern in the business community. One major issue is the resumption of inspections by the SSUFSCP, which, given the agency’s prior practices, has raised doubts about the transparency and quality of such oversight. Another concern is the impact on small pharmacy businesses. These businesses operate within familiar profit margins, and the new regulations reduce those margins — potentially prompting some to close pharmacies in smaller towns and rural areas, where profits are limited but fixed costs remain. Moreover, reduced profitability and restrictions on traditional pricing models could make it economically unviable for some businesses to continue offering certain types of medicines, potentially leading to reduced availability of those drugs on the market.
The pharmaceutical sector remains resilient, as public demand for medicines is consistently high. However, an open and constructive dialogue between the government and the pharmaceutical industry is now more important than ever. Businesses need to analyze the economic implications of the resolution in order to engage with authorities based not on emotion, but on data and evidence.”
Authorization to use patented medicines without the patent holder’s consent in case of market shortages: +1 point
The Cabinet of Ministers has updated the procedure for granting pharmaceutical manufacturers permission to use patented inventions related to medicines. Previously, such permission could be issued only with the consent of the patent holder. Under the new rules, however, the government may grant a license without consent during times of war, a state of emergency, or an epidemic — but only if there is a shortage of the relevant medicine and the patent holder is not supplying the market and refuses a request from the Ministry of Health or an applicant to authorize its production. At the same time, the resolution strengthens the protection of patent holders’ rights. Whereas previously a license could be granted at any time, it is now limited to clearly defined exceptional circumstances.
The updated procedure allows Ukrainian manufacturers or importers of medicines to receive such a license, which is valid only within the territory of Ukraine. The patent holder must be compensated financially for the use of their invention without consent. The quantity of medicines produced may not exceed domestic demand, and the products must be clearly labeled to indicate that they were manufactured under special government authorization. Importantly, even after the government grants another company permission to use a patented invention, the patent holder retains the right to independently license it to others.
Expert commentary
Ilona Sologoub, Scientific Editor at Vox Ukraine
“The document amends Cabinet Resolution No. 877 of December 4, 2013, ‘On the Approval of the Procedure for Granting Permission by the Cabinet of Ministers of Ukraine to Use Patented Inventions (Utility Models) Related to Pharmaceutical Products.’ The resolution allows the Cabinet to authorize Ukrainian manufacturers to produce patented medicines without the consent of the patent holders, provided that the latter are appropriately compensated.
The new version of the resolution somewhat improves intellectual property rights protection for the patent holders. Specifically, the Cabinet may now grant permission only during a state of emergency or quarantine, and only if the patent holders are unable to meet Ukraine’s demand for the medicines — which could result in a shortage of at least 51% over a six-month period. Previously, simply citing unmet demand for the medicines was sufficient to obtain such permission.
The revised resolution stipulates that medicines produced under permission granted by the Cabinet of Ministers may be used exclusively on the domestic market. It also gives the patent holder the opportunity to object to the granting of such permission, as the Cabinet is now required to publish an announcement on its official website at the start of the licensing procedure. Before initiating this process, the Ministry of Health must first contact the patent holder with a proposal to negotiate the terms of use for the patent. As a result, the possibility of using intellectual property without the consent of the rights holder will be more restricted — a change expected to enhance Ukraine’s reputation in the area of intellectual property rights.”
Oksana Kuziakiv, Executive Director at the Institute for Economic Research
“The resolution allows for the use of patented medicines without the consent of the patent holders, provided that the user does not seek to make a profit. This mechanism, known as compulsory licensing, is an important tool for ensuring access to life-saving medicines. It is particularly relevant for Ukraine in the context of war and limited resources.
A key issue is the need to align Ukraine’s mechanism with that of the European Union, which is currently developing a new framework for regulating compulsory licensing in emergency situations. One of the proposals under consideration in the EU stipulates that products manufactured under such licenses must remain within the EU and may not be exported to non-member countries — including Ukraine. This raises concerns that Ukraine may face difficulties: even after implementing its own mechanism, if it is not recognized by or coordinated with the EU’s official system, the two frameworks could operate independently, with no possibility of mutual export. To prevent such challenges, a systematic dialogue is needed.”
Olga Gurgula, Lawyer, Senior Lecturer in Intellectual Property Law at Brunel University London
“It is a positive development that lawmakers have initiated reform of the compulsory licensing mechanism — particularly with regard to pharmaceuticals — as the current procedure for issuing compulsory licenses for medicinal products is largely non-functional.
The resolution addresses several problems. For instance, in cases where the patent holder either refuses to grant a license or fails to respond within 30 calendar days from the date of the request, the Ministry of Health may now initiate the licensing procedure. Under the previous version, a license could not be issued if the right holder simply failed to respond.
However, the procedure still has a number of shortcomings. One is the vague wording of the first condition under which a license may be granted — namely, when ‘the patent holder is unable to meet the need for the relevant medicinal product using the facilities and capacities ordinarily used for the production of such a product in Ukraine, which would lead to a significant shortage of that product.’ This formulation lacks clarity and may be difficult to apply in practice. In addition, the regulation effectively prohibits export. The provision stating that the invention may be used only for the domestic market is inconsistent with both international and current Ukrainian legislation. Such a restriction is not present in either Article 31 of the TRIPS Agreement (which regulates compulsory licensing) or Article 30 of the Ukrainian Law on the Protection of Rights to Inventions and Utility Models — both of which provide that use should primarily serve domestic needs.
Another important shortcoming is that a compulsory license for a patent alone may not be sufficient for effective technology transfer. For example, when it comes to complex biological products such as vaccines, protection is afforded not only by patents but also by trade secrets. At present, Ukraine lacks an effective mechanism for the compulsory transfer of trade secrets. This factor must also be considered when developing a new compulsory licensing framework.”
Reform Index from VoxUkraine aims to provide a comprehensive assessment of reform efforts by Ukraine’s authorities. The Index is based on expert assessments of changes in the regulatory environment in six areas: Governance, Public Finance, Monetary system, Business Environment, Energy, Human Capital. Information about the Reforms Index project, the list of Index experts and the database of the regulations assessed are available here.
Attention
The author doesn`t work for, consult to, own shares in or receive funding from any company or organization that would benefit from this article, and have no relevant affiliations



