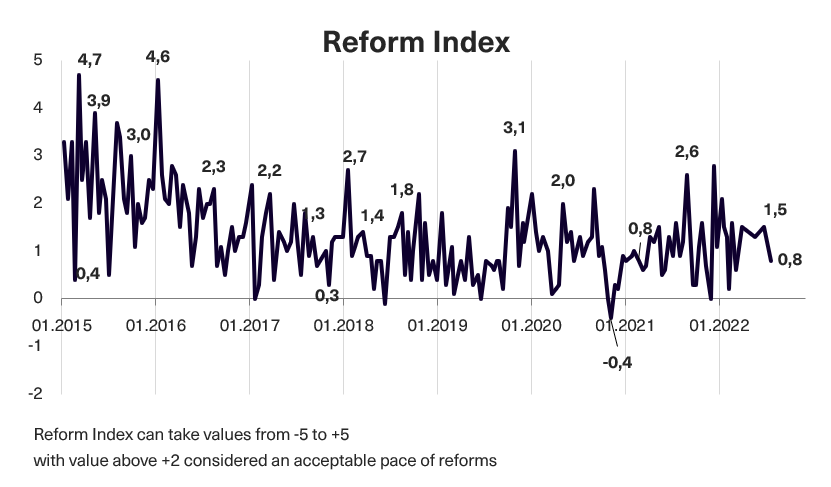
In issue 187-188 (issue 184 before the audit) of the Reform Index, 13 changes have been included that are capable of influencing the “rules of the game” in the country. Of them, only two received +2 points from experts, which we think separates significant reforms from less important ones. These are the Law on Administrative Procedure and the Law on Compassionate Use of Medicines. Overall, from May 23 to June 19, 2022*, the Reform Index is +0.8 points, with values ranging from -5.0 to +5.0. In the previous round, the Index was +1.5 points. Read more below.
*In connection with the war in Ukraine and the state’s change of focus to wartime needs for the period of active hostilities, we changed the period of monitoring regulations for the Index for Monitoring Reforms from two to four weeks.
Chart 1. Reform Index Dynamics
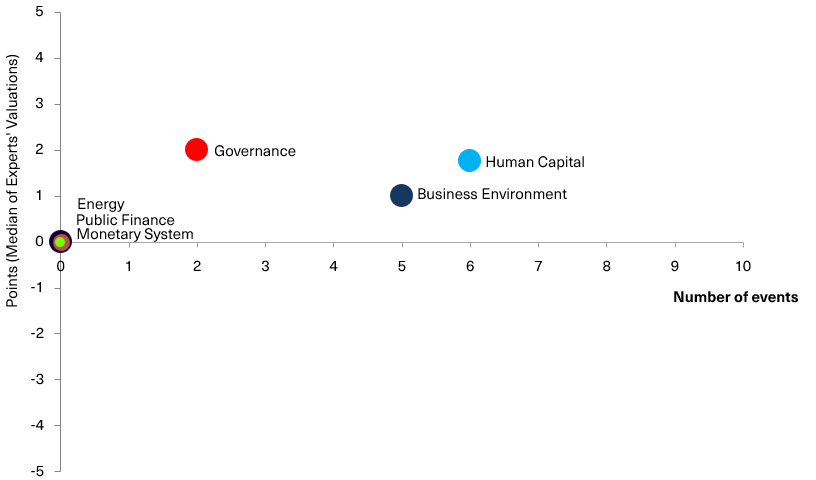
Chart 2. Reform Index and its components in the current round
Law on administrative procedure: +2.0 points
Law 2073-IX creates a framework for the relationship between executive authorities, citizens, and legal entities. It implements the principles of administrative procedure, including the rule of law, equality before the law, the presumption of legality of a person’s actions, timeliness, and guaranteeing the person a right to participate in administrative proceedings.
Significant innovations include the introduction of the “interested persons” category for administrative cases and the requirement for authorities to notify such persons about the initiation of administrative proceedings related to their interests. Also, persons adversely affected by an administrative decision have the right to be heard before passing an administrative act with such a decision.
The law was signed on the second attempt. The Verkhovna Rada first voted on it in November 2021. However, Volodymyr Zelensky vetoed the bill proposing to exclude from the law’s purview relations concerning national security, defense, and granting citizenship or asylum in Ukraine. His proposals were taken into consideration, and the president signed the law in June this year.
Information about the Reforms Index project, the list of Index experts and the database of the regulations assessed are available here.
Law on compassionate use of medicines: +2.0 points
Law 2054-IX enables seriously ill patients to receive free drugs not registered in Ukraine.
The mechanism will be available under two types of programs:
- a program granting patients access to investigational drugs after completing clinical trials;
- a program granting patients expanded access to unregistered medicines (e.g., drugs undergoing at least phase II clinical trials in the USA, EEA countries, Australia, Canada, Japan, Great Britain, Israel, and Switzerland).
To participate in the programs, several conditions need to be met: the availability of data on the ratio of risks and benefits when using the drugs, patient with severe medical conditions, the lack of access to other effective treatment methods on the territory of Ukraine, or ensuring the continuity of treatment with certain drugs following the end of participation in clinical trials.
The Ministry of Health is the body authorizing extended access programs. The MoH’s authorization means that unregistered medicinal products are permitted to be imported into the customs territory of Ukraine.
Similar programs are already in place in the world’s most developed countries, particularly the U.S., Great Britain, Denmark, and other countries.
Chart 3. Value of Reform Index components and number of events
Reform Index from VoxUkraine aims to provide a comprehensive assessment of reform efforts by Ukraine’s authorities. The Index is based on expert assessments of changes in the regulatory environment in six areas: Governance, Public Finance, Monetary system, Business Environment, Energy, Human Capital.
This publication was produced within the framework of the “Support of think tanks” project which is carried out by the International Renaissance Foundation with the financial support of the Embassy of Sweden in Ukraine. Its contents are the sole responsibility of the authors and do not necessarily reflect the views of the Embassy of Sweden in Ukraine and the International Renaissance Foundation.
Attention
The author doesn`t work for, consult to, own shares in or receive funding from any company or organization that would benefit from this article, and have no relevant affiliations